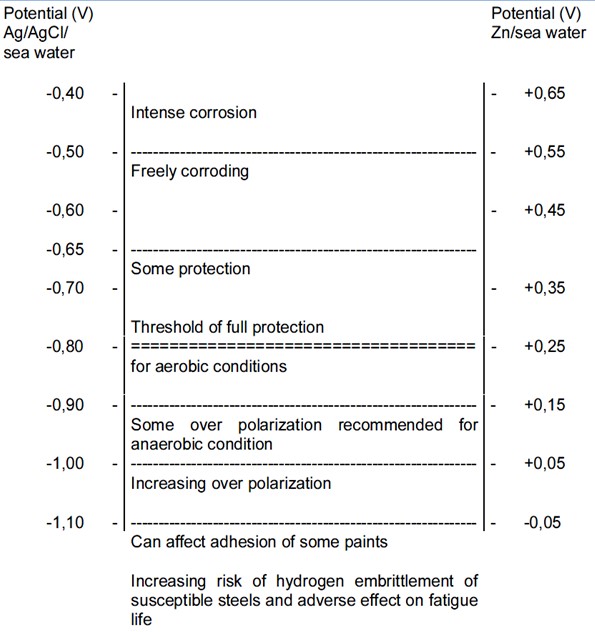
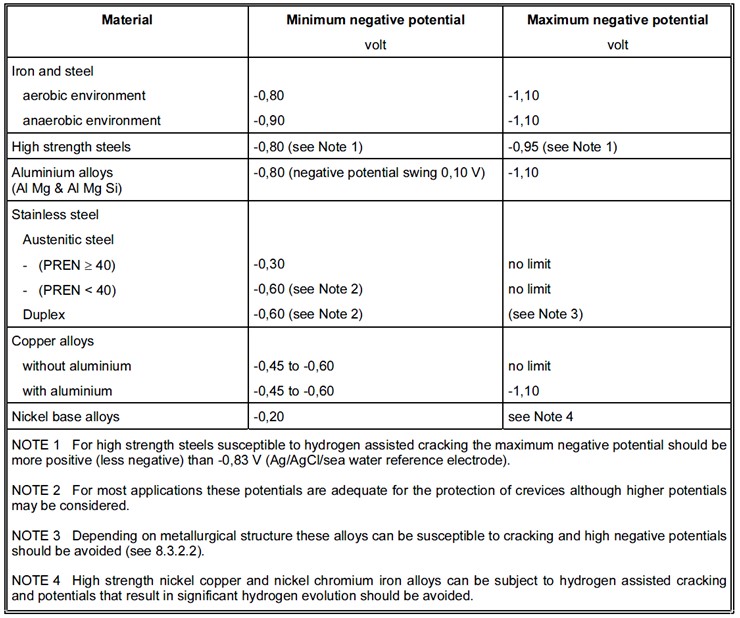
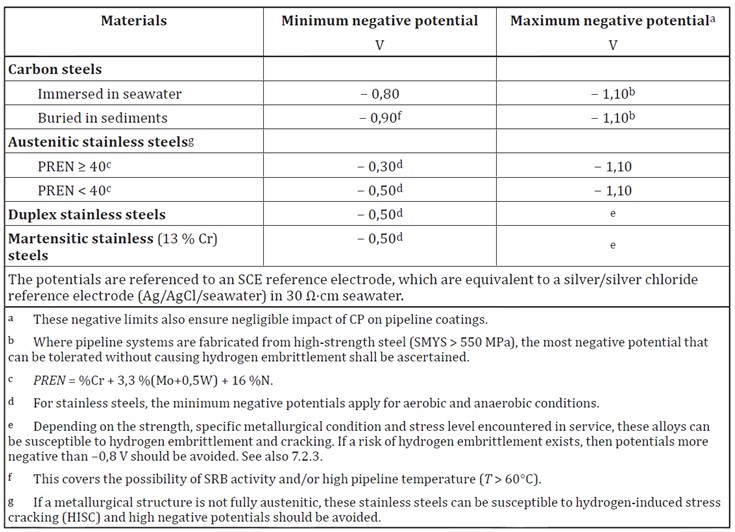
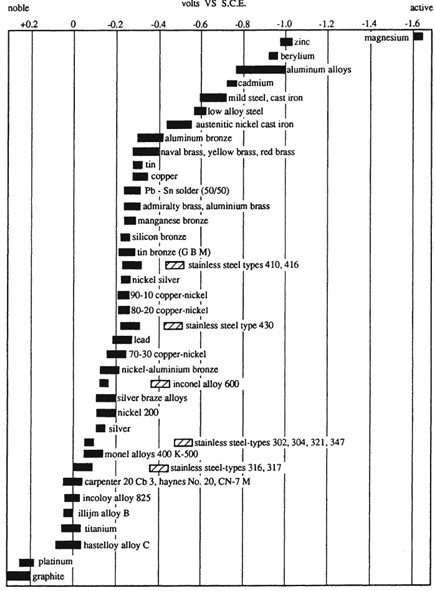
The cathodic protection potential is the potential which is required to be induced to protect the metal from corrosion. The cathodic protection potential is dependent on the type of metal being protected and on the environment. A summary of the protective potentials of common marine materials taken from BS EN 12473 is presented at the bottom of this page.
The CivilWeb Cathodic Protection Design Spreadsheet can be used to complete the anode design for any cathodic protection potential. The designer simply inputs the required protection potential and the anode potential and the spreadsheet will calculate the required anode sizes. Note that the more negative the protection potential required, the smaller the effective cathodic current density achieved from the anodes. So a greater cathodic protection potential will require larger or more anodes. Also note that a more negative cathodic protection potential can have other undesired effects as explained below.
Cathodic Protection Potential - Carbon Steel
It is recommended that a value of -0.80V (Ag/AgCl/Sea Water reference electrode) is required to reduce corrosion of carbon steel to an acceptable level in sea water.
In anaerobic conditions where sulfate reducing bacteria are active, some standards recommend that the protective potential is reduced to -0.90V (Ag/AgCl/Sea Water reference electrode). This would generally apply to areas where Accelerated Low Water Corrosion has been identified. However a correctly designed cathodic protection system aimed at -0.80V will maintain a protective potential of between -0.90V and -1.05V for the majority of its design life. Only towards the end of the cathodic protection systems design life (around 80%-85%) will the potential rise above -0.90V and eventually rise above -0.80V leaving the steel under-protected. For this reason some standards do not regard the protection potential as a variable unless protective potentials more negative than -0.90V are aimed for.
There can also be detrimental effects on the properties of the steel if the potential drops too far. Some coatings can start to blister, there are adverse effects on the fatigue properties of the steel and some steels can become susceptible to hydrogen embrittlement.
For this reason it is normal to restrict the protection potential on mild steel to -1.10V, which is around the maximum that can be achieved on bare steel in sea water with aluminium anodes. For high strength steels with yield strengths greater than around 700N/mm2, the risk of hydrogen induced stress cracking means that the protection potential needs to be limited to around -0.83V. Therefore in areas where microbial corrosion or anaerobic conditions may be present, it is advised that coatings or biocides are used in place of traditional cathodic protection systems.
The effects of different protection potentials on steel in sea water are shown in the below diagram taken from BS EN 12473.
Cathodic Protection Potential - Aluminium Alloys
As well as steel, other metals are commonly used in the marine industries and may require cathodic protection systems. Aluminium alloys are often used due to their lightness and natural corrosion resistance. Aluminium forms a very protective oxide film on the metals surface which is highly inert and resistant to corrosion. Despite its natural resistance to corrosion, aluminium alloys can be protected with cathodic protection systems if required.
The potential of aluminium magnesium and aluminium magnesium silicon alloys in sea water is generally in the range of -0.70V and -0.90V. They can be cathodically protected with a negative potential swing of 0.1V from the corrosion potential.
The negative limit of aluminium alloys is generally taken as -1.10V. Higher negative potentials should be avoided particularly in stagnant conditions as the alkali generated may strongly attack the aluminium to produce the aluminate. Zinc anodes and the Aluminium/Zinc/Indium or Aluminium/Zinc/Tin anodes can be used. Aluminium anodes containing mercury should be avoided.
Cathodic Protection Potential - Stainless Steels
Stainless steels are prone to pitting corrosion in sea water. This appears to be related to the water pH, temperature and dissolved oxygen content. The steels resistance to pitting corrosion is evaluated using the Pitting Resistance Equivalent Number (PREN). PREN is related to the chromium, molybdenum and nitrogen content of the steel. The PREN can therefore be derived from the steels composition and can be calculated using the below equation;
Where the PREN is greater than 40, the steel is unlikely to suffer from pitting or crevice corrosion in sea water at 20°C.
Austenitic stainless steels can generally be protected from corrosion with a potential of -0.60V. For more corrosion resistant steels with a PREN of at least 40 this can be reduced to -0.30V. They do not generally need a limit to the protective potential.
Duplex stainless steels are largely resistant to general corrosion in sea water but can be susceptible to crevice and pitting corrosion, particularly at elevated temperatures. Protection potentials of around -0.60V are suitable to prevent this corrosion.
Duplex stainless steels are vulnerable to cracking at highly negative potentials as they contain too much ferrite which can be affected by hydrogen embrittlement. A negative limit of around -1.05V is recommended though this will depend on the steels composition and condition.
In systems where stainless steels are in electrical contact with carbon steels the two materials should be polarized to the same potential to avoid any galvanic corrosion couples forming.
Cathodic Protection Potential - Copper Alloys
Copper alloys are often used as corrosion resistant claddings and in subsea completion units and well closure assemblies.
Copper alloys have excellent corrosions resistance in sea water and even discourage marine growth. For this reason copper components do not generally require cathodic protection. However copper components which have cathodic electrical continuity to cathodically protected steel components will receive protection and this can actually remove the barriers to marine growth present in unprotected copper.
The current densities required to protect copper alloys or copper/steel couples can be much higher than the current densities required for the protection of the steel alone. This is particularly the case when the copper does not have an oxide layer. However, where the corrosion rate is primarily controlled by the reduction of dissolved oxygen in the sea water, then the required current density will not exceed the limiting current for dissolved oxygen reduction.
Cathodic Protection Potential - Nickel Alloys
Nickel copper alloys are commonly used for the corrosion resistant cladding of steel components and as such as usually electrically connected to large areas of cathodically protected steel. While this will polarize the nickel components to a potential of around -0.80V to -1.10V, the a protective potential of around -0.20V would be sufficient for the nickel components alone.
There have been some cases of hydrogen embrittlement in high strength nickel copper and nickel chromium iron alloy components. Where this may be applicable further testing may be required to check that the nickel alloys will not be affected by hydrogen embrittlement at the design potentials for the structure.
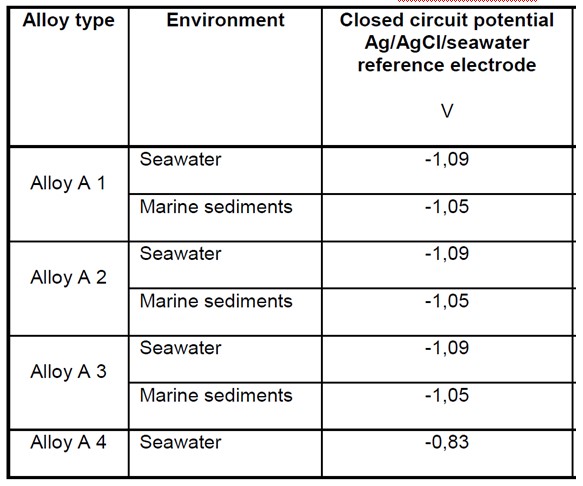
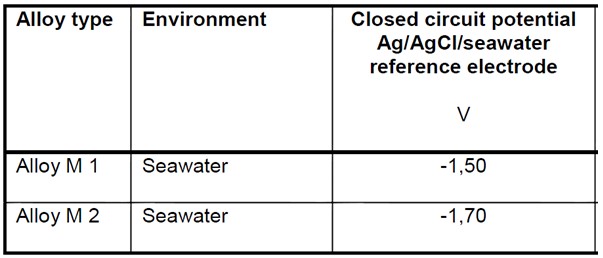
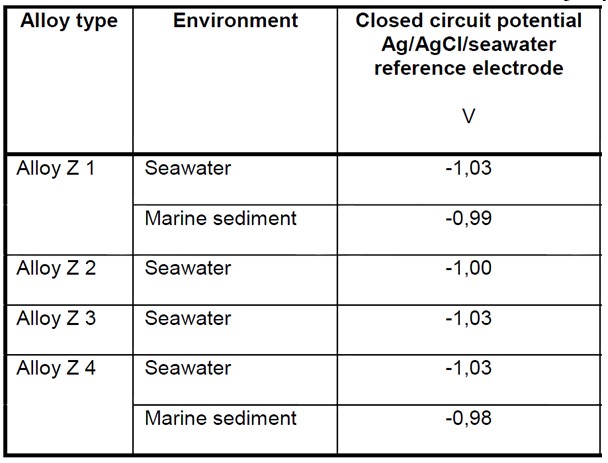
Anode Closed Circuit Potential (E’a)
The Anode Closed Circuit Potential is used to determine the Driving Voltage of the galvanic cell created when the anode and cathode are connected in the electrolyte. The difference between this value and the design cathodic protection potential represents the Driving Voltage of the anode, which along with the anode resistance to the electrolyte determines the cathodic protection current density of the anodes.
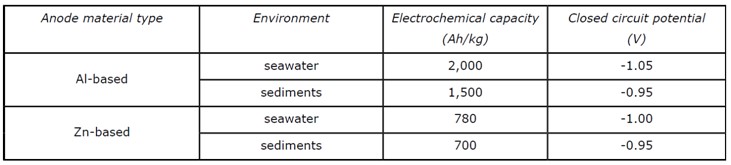
The Closed Circuit Potential of a particular alloy should be taken from the manufacturer or from long term testing or experience of the alloys performance in service. Where this information is not available standard values can be taken from national standards. Some of these are reproduced at the bottom of this page.
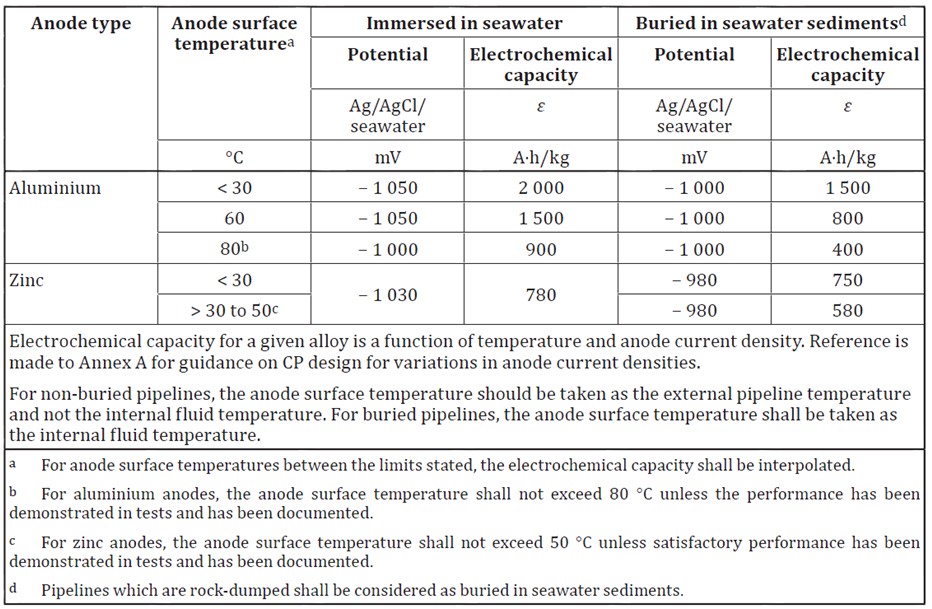
It should be noted that the specific performance onsite can be influenced by the cathodic current density and by the operating temperature. Aluminium alloys will experience a dip in potential at temperatures over 60°C, and caution should be exercised for any applications over 80°C unless specific performance testing is to be undertaken or long term experience in similar conditions is available.
Anodes should be tested for their electrochemical capacity in accordance with the standard testing procedures. It is recommended that at least one short term test is undertaken for every 15,000kg of anodes produced. In these short term tests it is expected that at the end of the 4th testing period the anodes will achieve values more negative than -1.05V for aluminium based anodes and -1.00V for Zinc based anodes.