The precise anode composition is important as this will determine the electrochemical properties of the anode. This information will be given by the manufacturer and should be checked with the testing of samples. The CivilWeb Cathodic Protection Design Spreadsheet can be used with any composition of anode, the designer simply inputs the design parameters such as the electrochemical capacity and anode density for the chosen anode composition and the spreadsheet will calculate the performance.
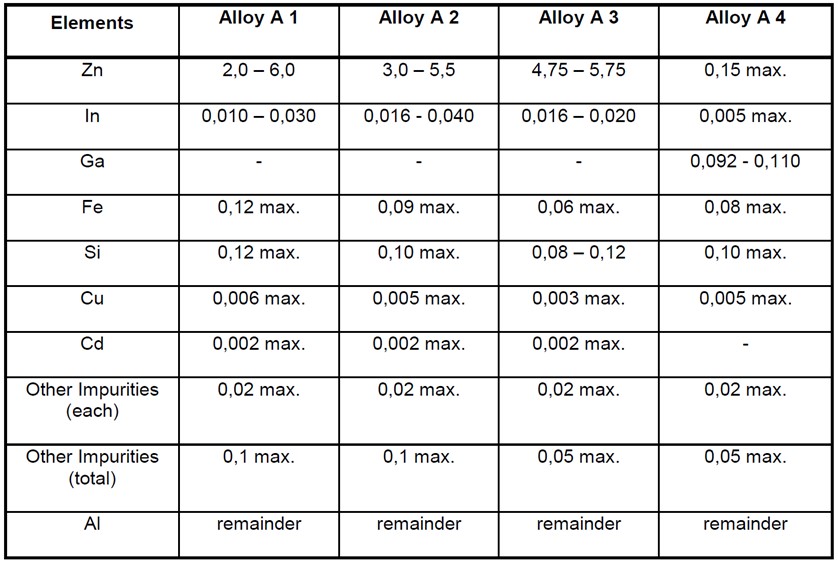
Aluminium Alloy Anode Composition
Aluminium based anodes usually contain zinc and a small amount of indium which acts as an activator. The precise makeup of the alloy must be closely controlled as some aluminium alloys have been found to produce a protective film which prevents the anode from dissolving and providing the required current output.
The following alloys have been found to be useable in marine seawater and sediments;
The above table is taken from BS EN 12946. Alloys A1 is commonly used for general marine purposes, Alloy A2 for offshore applications, Alloy A3 for deepwater and cold water applications, and Alloy A4 is used for low driving voltage applications such as for high strength steel.
Note the seawater must have a salinity greater than 0.5% for aluminium alloys to be usable.
Other Constituents
Magnesium is no longer included as a component in aluminium based anodes as these alloys can suffer from age hardening and cracking.
Mercury is no longer included due to environmental and safety concerns.
Alloys containing tin require heat treatment to achieve effective activation and can be affected by corrosion and slow activation in cold conditions.
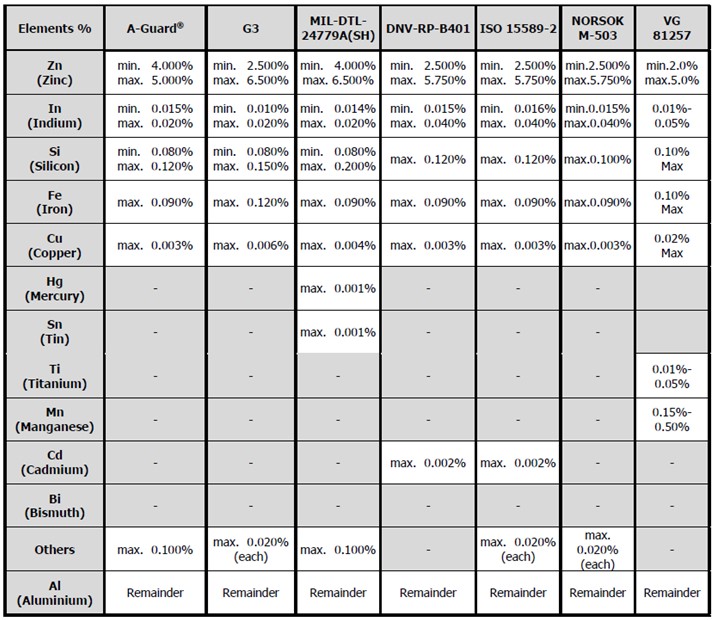
The following table shows a comparison of several international standard compositions for aluminium alloy anodes;
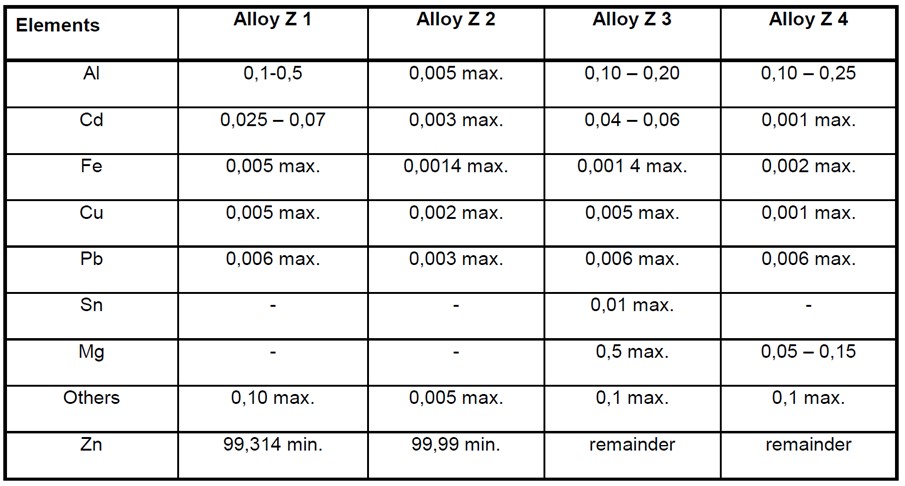
Zinc Alloy Anode Composition
Zinc based alloys are widely used for shipping and offshore applications. The use of zinc anodes is limited when there are weight restrictions due to their high density. Zinc anodes can also be used in fresh water and brackish water where the salinity is not high enough for aluminium anodes.
Pure zinc anodes are sometimes used but they can only be used if the iron impurity content is less than 0.0014%. This impurity level can be increased with the addition of aluminium to the alloy.
The following table taken from BS EN 12496 shows the composition of common zinc alloys used for anodes;
Alloy Z1 is usually supplied in accordance with US MIL-A-18001-K or to ASTM B418 type 1.
Alloy Z2 is a high purity zinc alloy and is normally supplied in accordance with ASTM B418 type 2.
Alloy Z4 is generally used at elevated temperatures.
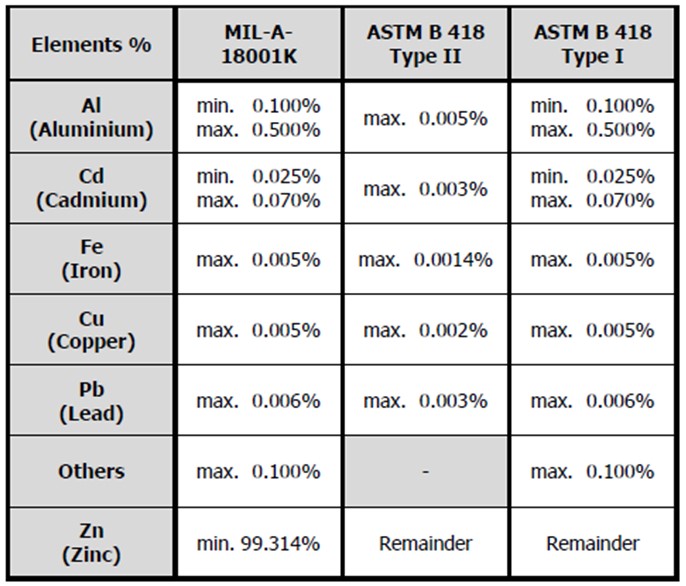
The following table shows a comparison of several international standard compositions for zinc alloy anodes;
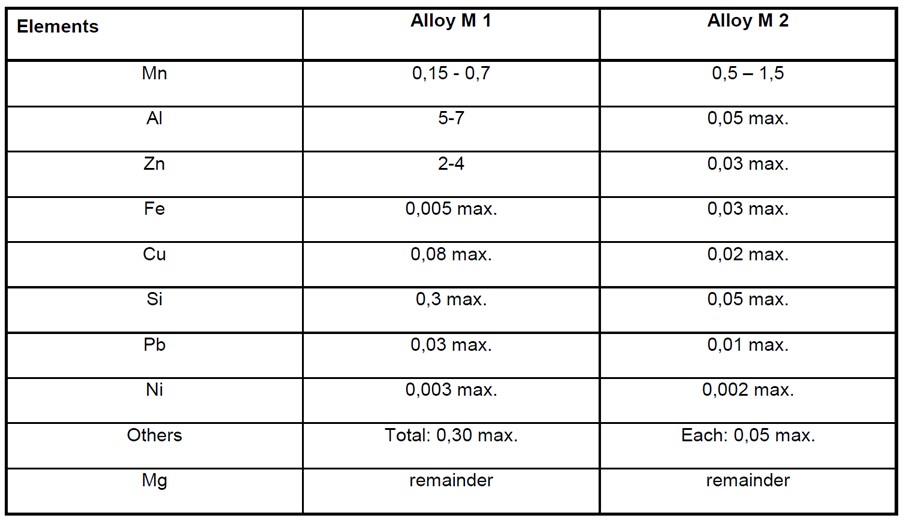
Magnesium Alloy Anode Composition
Magnesium based alloys are not usually used in seawater as the low resistivity of seawater makes aluminium or zinc alloys more efficient. However they are sometimes used in fresh or brackish water where a high driving voltage is required. They are also sometimes used in seawater when rapid polarization is required. Due to their highly negative potential and high consumption rate magnesium alloys are usually used where frequent anode replacement is possible.
The following table taken from BS EN 12496 shows the composition of common magnesium alloys used for anodes;
For both of these alloys there is a need to control the naturally occurring residual impurities which can accelerate self-corrosion. Manganese is sometimes added to sequester iron impurities and to make the potential of the anode more negative.