The electrochemical capacity of the anodes is determined by the precise anode composition, though typical values are sometimes used for preliminary design. Some typical values for common anodic materials are given at the bottom of this page. The CivilWeb Cathodic Protection Design Spreadsheet can be used to design cathodic protection systems using any anodic material. The designer simply inputs the electrochemical capacity of the material and the spreadsheet calculates the mass of anodic material required to meet the design cathodic protection potential.
Anode Eletrochemical Capacity
After the anode potential is known for the initial and final stage calculations, the next step in anode design is to calculate the anode electrochemical capacity which is critical for the maintenance stage calculations. The electrochemical capacity of the anodes is the total amount of electricity generated when 1kg of the anode is consumed. This value depends on the anodic material and on the precise composition of the anode, the cathodic current density, the operating temperature and other environmental factors.
This value can be taken from the manufacturer but care should be taken when using this value as it will often be based on short term tests. This involves a higher current density being applied to the anode which returns values over 2,500 Ah/kg for aluminium anodes which is close to the theoretical Faraday limit for the material. This does not mimic the conditions in service as self-corrosion in operational conditions reduces the practical electrochemical capacity of the anodes. A better indication of a particular alloys performance should be taken from either long term performance tests of at least 12 months duration or from long term practical experience. Details of testing procedures are given on our anode manufacture page.
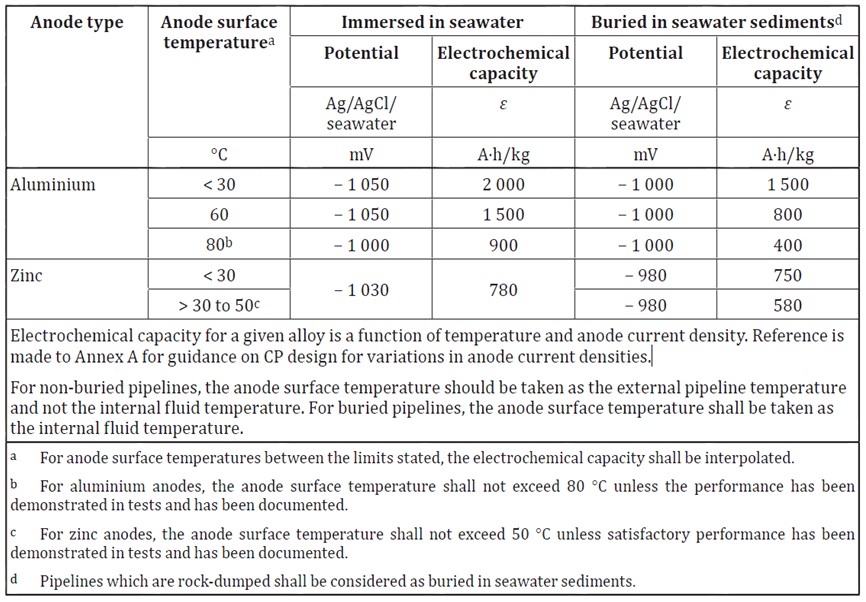
Where long term experience or testing is not available, there are a number of recommended values given in national standards. Caution should be exercised when using these values for design, particularly in the European standards. For preliminary design the below values given in ISO 15589-2 are conservative.
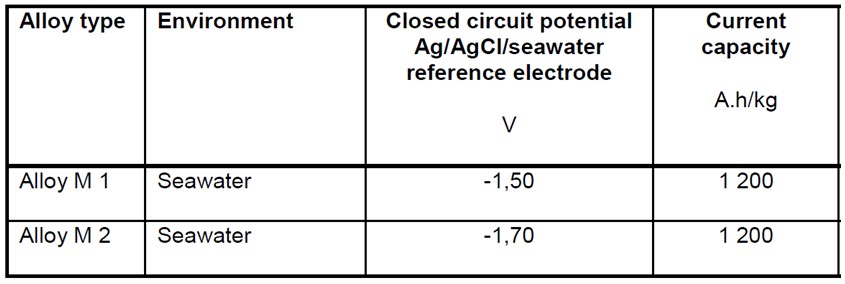
Magnesium anodes are sometimes used because of their high closed circuit potential but they have a low electrochemical capacity, as shown in the below table taken from BS EN 12496.
Anodes should be tested for their electrochemical capacities. It is recommended that at least one short term test is undertaken for every 15,000kg of anodes produced. In these short term tests it is expected that the anodes will achieve values in excess of 2,500 Ah/kg for aluminium based anodes and 780 Ah/kg for Zinc based anodes. These values are suitable when using the conservative design values stated above. If a higher design value is being used then higher test results may be expected.
Factors Affecting the Anode Electrochemical Capacity
Aluminium based anodes will experience a significant drop in electrochemical capacity at high operating temperatures. The anodes electrochemical capacity will drop in a fairly linear fashion between 2,000Ah/kg at 30°C to around 900 Ah/kg at 80°C, as shown in the above table. Temperature values between these can be ascertained by linear interpolation. Note that aluminium anodes at operating temperatures above 80°C will need to be tested to ascertain their performance.
The electrochemical capacity of anodes is also affected by the salinity of the water. The above values assume a salinity of around 3.5%. If the water salinity is lower than 1.2% the values for anode performance in marine sediments may be more applicable than the seawater values.
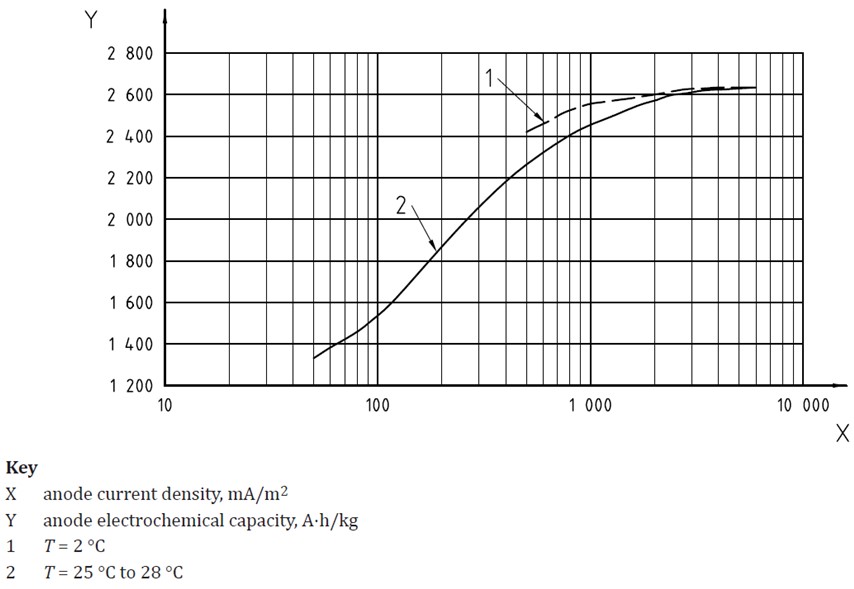
Another factor influencing the electrochemical capacities achieved in practice is the current output. At very low current outputs the electrochemical capacities are reduced, as shown in the below graph taken from ISO 15589-2.